
Product classification
The scope of FEFANA covers specialty feed ingredients and their mixtures. Specialty feed ingredients comprise feed additives and functional feed ingredients.
Feed additives
Feed additives are substances, micro-organisms or preparations which are intentionally added to feed or water in order to perform one or more of the functions described in Article 5 of Regulation (EC) No 1831/2003 on additives for use in animal nutrition.
Premixtures
Premixtures are mixtures of feed additives or mixtures of one or more feed additives with feed materials or water used as carriers, not intended for direct feeding to animals.
The EU legislation puts the basic responsibility to properly classify a feed product into one or another category on each operator. This choice and the resulting legal obligations are then controlled by the local competent authority in charge of official controls at the national level.
It is pivotal for each operator to make the right choice in order to avoid any market disruption. With the objective to help the industry and the control authorities to manage product classification in a fair and predictable way, the EC has adopted guidelines for the distinction between different types of feed (e.g. feed additives vs. feed materials),
which should create the necessary transparency in order to manage product classification in a fair and predictable way.
On top of that, FEFANA has developed a user-friendly Classification Tool in order to implement these guidelines. Based on very simple questions, this tool allows the allocation to the proper regulatory classification status. Such a tool is proved helpful for operators to ensure compliance of their products with regulatory frameworks. It is also used by the authorities to assure a consistent regulatory management of such products on the market.

Feed additives are substances, micro-organisms or preparations which are intentionally added to feed or water in order to perform one or more of the functions described in Article 5 of Regulation (EC) No 1831/2003 on additives for use in animal nutrition. All feed additives placed on the market in the European Union must be approved under the auspices of the mentioned regulatory framework.
The principal aim of this regulation is to ensure that all additives approved for use in the EU are safe not only for the target animal, but also for those involved in its handling, the ultimate human consumer of the animal products, as well as the environment.
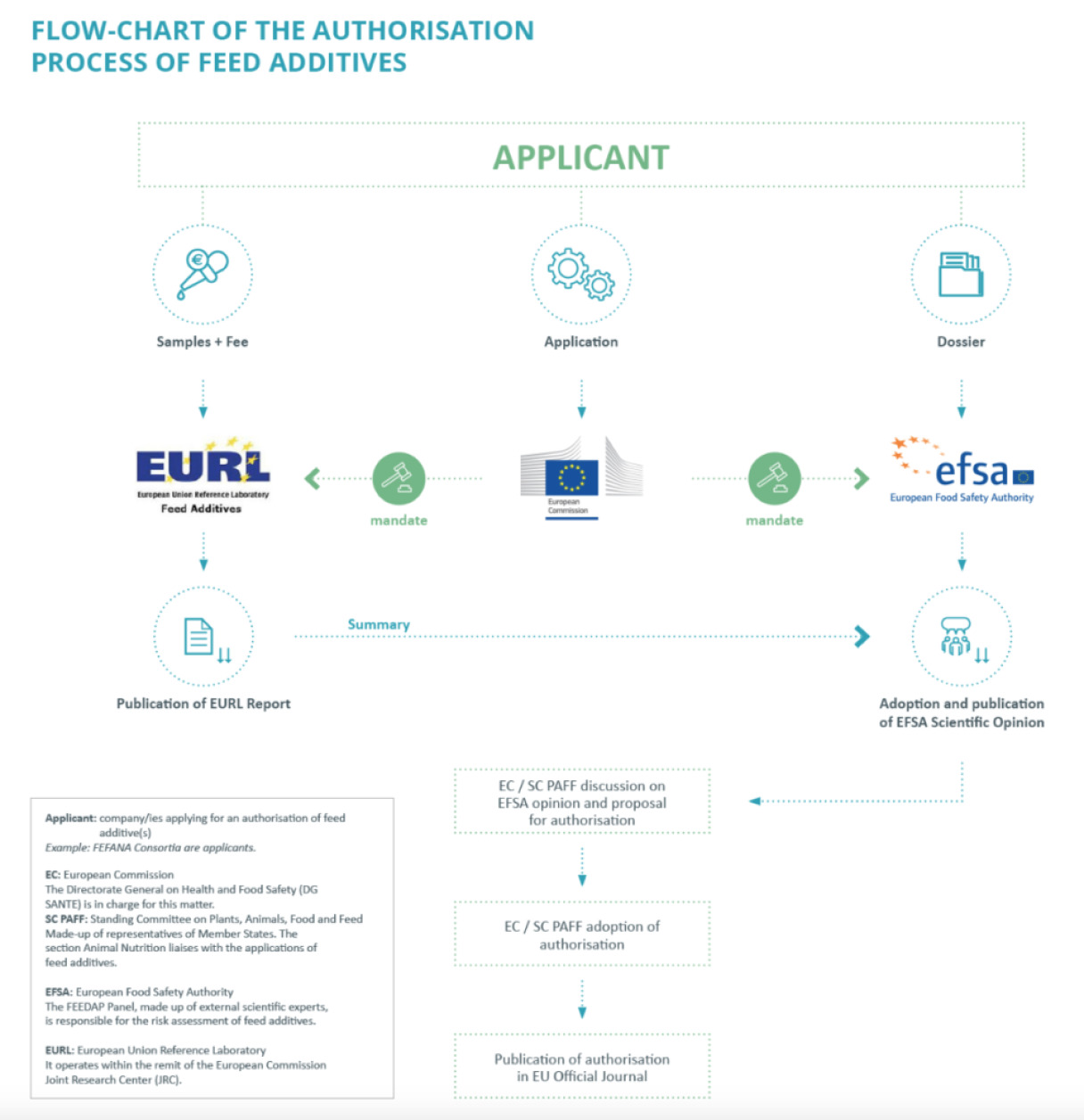
As a result, each additive has to undergo a series of meticulous tests to demonstrate it is safe to use and handle, and data is required to demonstrate the efficacy of the additive for its intended use before it can be marketed in the EU. Such data is presented in the form of a comprehensive dossier which is forwarded to and assessed by the European Food Safety Authority (EFSA). Subsequently, the dossier is voted upon by the European Commission. If the additive is approved, its name and conditions of use are put into the feed additive register, which is a public document. The procedure is therefore structured in a way to guarantee transparency and safety.
Functional feed ingredients are not legally defined in the EU, they are considered as feed materials that provide a benefit beyond basic nutrition, Thus, a feed material can also exert an additive function (e.g. as a thickener) but this should not be the only intended use[1]. Feed materials are defined in Regulation (EC) No 767/2009. Functional feed ingredients like other feed materialsdo not require pre-market approval.
Operators placing on the market functional feed ingredients are the only responsible to meet the requirements of EU legislation including Regulation (EC) No 178/2002, known as the General Food Law, and Regulation (EC) No 183/2005 on feed hygiene.
A clear distinction between feed materials, feed additives, and other categories is essential for regulatory compliance. The lawful marketing and labelling of these products depend on their correct classification in accordance with applicable legislation. Misclassification may result in non-compliance with regulatory requirements, leading to market withdrawal.
[1] 2011/25/EU: Commission Recommendation of 14 January 2011 establishing guidelines for the distinction between feed materials, feed additives, biocidal products and veterinary medicinal products
Premixtures are mixtures of feed additives or mixtures of one or more feed additives with feed materials or water used as carriers, not intended for direct feeding to animals.
The production and use of premixtures is ruled by Regulation (EC) No 1831/2003 on feed additives for use in animal nutrition.
A premixture is intended for incorporation in compound feedingstuffs, feed materials or water and not meant for direct feeding to animals. The function of the premixture is to optimise mixing of feed additives in feedingstuffs.
Want to join FEFANA?
Become a member.
Want to know more about FEFANA ?
Contact us



